On the morning of March 20, eye specialist Jason Comander infused infections conveying lab-developed qualities into the eyes of a kid whose vision had been slowly vanishing.
In the event that all goes as arranged, the 13-year-old patient who lives with an acquired hereditary deformity that causes visual impairment will encounter a change in vision in about a month.
After a progression of tests, the U.S. Sustenance and Drug Administration (FDA) endorsed this quality treatment, called Luxturna, in December 2017.
After three months, the strategy Commander performed at Massachusetts Eye and Ear filled in as the first run through a FDA-endorsed quality treatment was utilized on a man living with an acquired, and serious, hereditary illness. There are no other compelling medicines for this particular retinal infection.
“It’s an immense positive development,”
said Comander, relate executive of the Inherited Retinal Disorders Service at the Boston-based healing facility, in a meeting.
“The greater part of the patients are legitimately visually impaired by their 20s or 30s,”
said Comander.
“Do the trick to state, these are extremely appalling illnesses.”
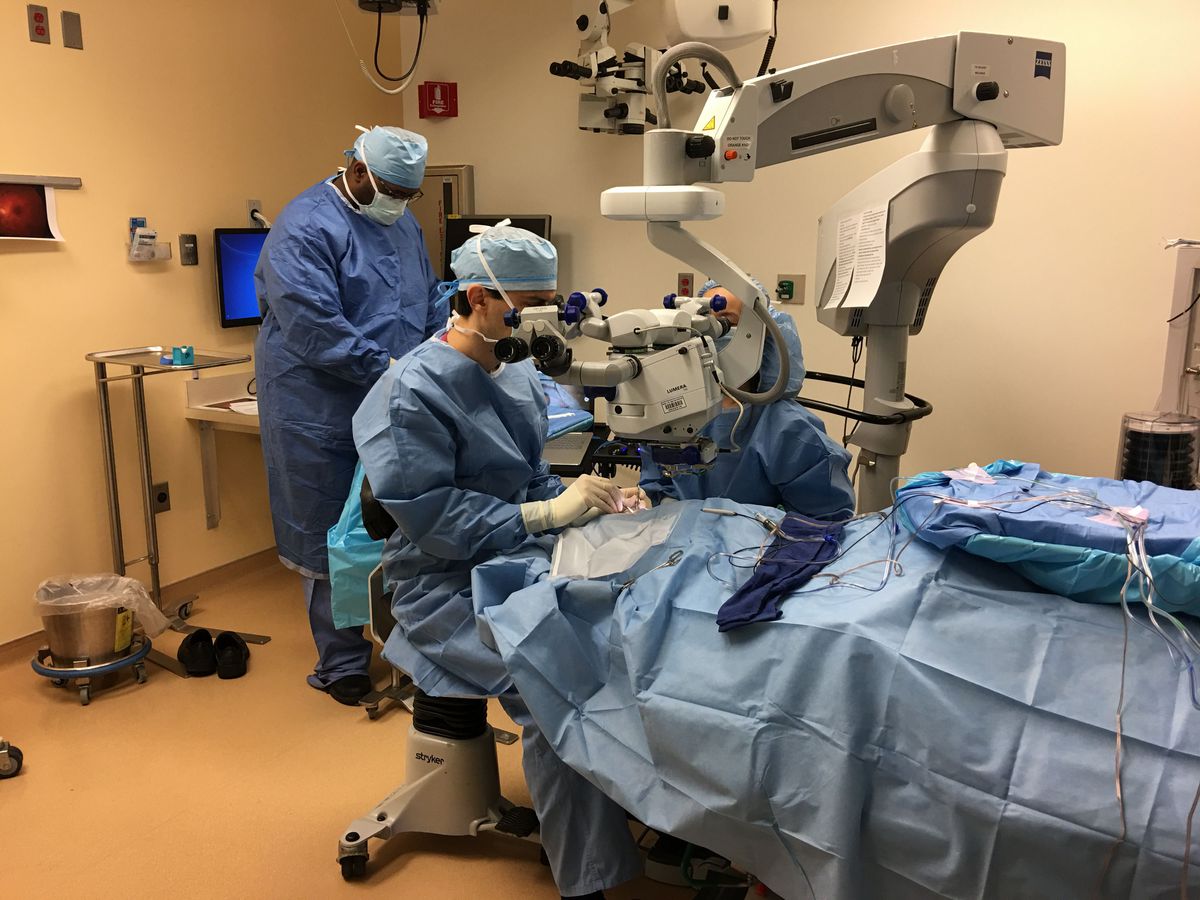
Researchers have been taking a shot at this novel sort of treatment for a considerable length of time, including as a major aspect of FDA trial programs. Comander takes note of the treatment influences diverse patients in various ways. Now and again the vision change is sensational. One past patient, from a prior FDA trial, never again expected to go to a specific school for kids with visual impairment, Comander said.
“This has been a fantasy of the field for a long time, and there have been calming mishaps,” said Tim Cherry, who investigates acquired visual impairment and other visual issue at Seattle Children’s Research Institute, in a meeting. Cherry uncovered he has teamed up with Comander on inquire about previously, yet had no impact in the advancement of this treatment.
“It can enhance individuals’ lives in a way that may have been considered sci-fi years back,”
said Cherry. It ought to be noted, in any case, that while daze individuals confront challenges because of unavailability, numerous live upbeat, satisfied lives.
The uncommon, transformed quality in beset patients, known as RPE65, keeps the retina, a light-delicate tissue behind the eye, from working accurately. In particular, the quality keeps retinal cells from legitimately creating proteins, bringing about falling apart visual perception. Numerous patients turn out to be legitimately visually impaired, and some lose all their vision.
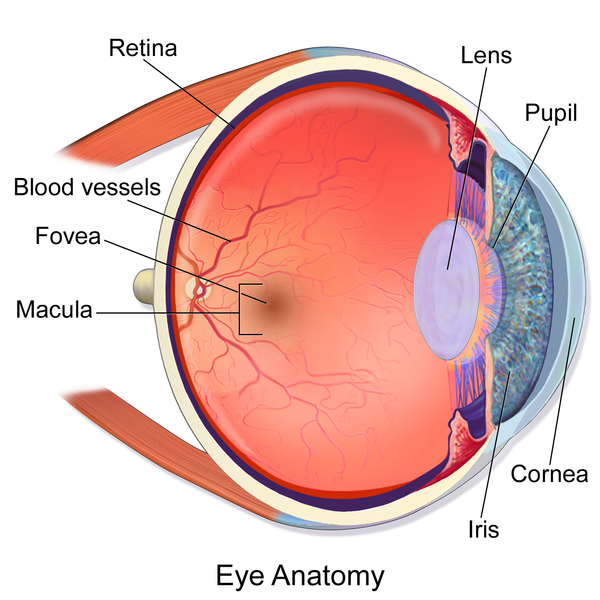
This novel quality treatment, called quality substitution treatment, is intended to supplant the changed qualities with qualities that work, and as needs be, enhance a patient’s visual perception. Engineered qualities are first developed in a lab and after that embedded into infections regularly found in the human eye. The infections — whose mission is to attack eye cells and implant hereditary material into them are then painstakingly put in the patient’s retina.
These infections, said Cherry, normally live in our eyes and are innocuous.
Since there were no other important medications accessible for this serious infection, the FDA endorsed the medicines more quickly than it commonly does, under projects called Breakthrough Therapy and Priority Review.
These sorts of quickened endorsements require medicate organizations and clinicians to keep observing the medicines after their underlying FDA endorsement, to guarantee they’re genuinely protected and sufficiently compelling for shoppers to utilize. The wellbeing notices on the Luxturna site underscore these potential perils, including potential eye diseases and further decreases in vision.
Prior in 2017, the FDA had endorsed two other quality treatment medicines for growths, which aren’t considered hereditarily acquired sicknesses. The FDA now gets ready for quality treatments to assume a more noticeable part in fighting beforehand untreatable sicknesses.
“I trust quality treatment will turn into a pillar in treating, and possibly curing, a significant number of our most decimating and immovable ailments,” FDA Commissioner Scott Gottlieb said while declaring the endorsement of Luxturna.
Orginal article by Mark Kaufman